Menu
Rx Generic Product Catalog
Therapeutic Class: Oral Contraceptive
TE Code:
AB
AB
Therapeutic Equivalence [TE] Code:
Products meeting necessary bioequivalence requirements
OTC/Rx:
Rx
Rx
Requires a Prescription
Important Product Resources:
Including Black Box Warning
and Patient Labeling
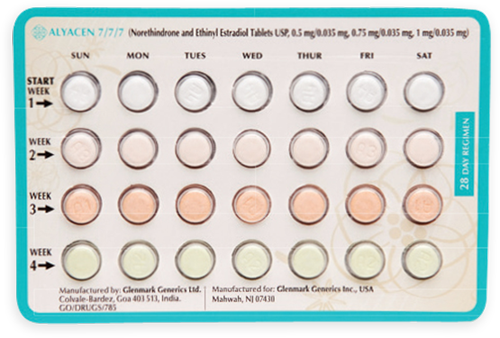
Strength: 0.5 mg/0.035 mg
Description:
(7)
White to Off-White,
Round Tablets [Norethindrone and Ethinyl Estradiol]
(7)
Light Peach,
Round Tablets [Norethindrone and Ethinyl Estradiol]
(7)
Peach,
Round Tablets [Norethindrone and Ethinyl Estradiol]
(7)
Light Green,
Round Tablets [Inert]
Available Sizes [NDC & Pack]:
68462-0556-29
3 x 28 Carton

Imprint Side
1: A4
Imprint Side
2: Plain
Description:
White to Off-White, Round Tablets [Days 1-7]
White to Off-White, Round Tablets [Days 1-7]
Strength: 0.5 mg/0.035 mg
Contains:
Norethindrone and Ethinyl Estradiol
Norethindrone and Ethinyl Estradiol

Imprint Side
1: A3
Imprint Side
2: Plain
Description:
Light Peach, Round Tablets [Days 8-14]
Light Peach, Round Tablets [Days 8-14]
Strength: 0.75 mg/0.035 mg
Contains:
Norethindrone and Ethinyl Estradiol
Norethindrone and Ethinyl Estradiol

Imprint Side
1: A1
Imprint Side
2: Plain
Description:
Peach,
Round Tablets [Days 15-21]
Strength: 1 mg/0.035 mg
Contains:
Norethindrone and Ethinyl Estradiol
Norethindrone and Ethinyl Estradiol

Imprint Side
1: A2
Imprint Side
2: Plain
Description:
Light Green, Round Tablets [Days 22-28]
Light Green, Round Tablets [Days 22-28]
Strength: n/a
Contains:
Inert
Inert




